|
"I am always ready to learn, but I do not always like
being taught." Winston Churchill
Part
1: Solve the following three
problems (you are not required to use an Excel spreadsheet)
Carbon Dioxide Removal #1
- A groundwater containing 20 mg/L of carbon dioxide
is to be degasified using a multiple-tray aerator with 5 trays. In
this water treatment facility there are 10 aerators operating in
parallel. For maintenance reasons only 8 of the aerators are available at
any one time. The design population is 40,000 persons, and the maximum day demand is
150
gal/person-day. The k value is 0.35, and the hydraulic
loading is 4 gpm/ft.2. Determine:
The carbon dioxide content of the product water.
The size of the trays if the length-to-width ratio is 2:1 and the trays
are made to 1 in. increments.
Solution: The performance equation is:
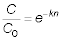
Therefore: C = (20 mg/l)e-5(0.35) = (20
mg/l)(0.174) = 3.48 mg/L
The flowrate to each parallel aerator is:
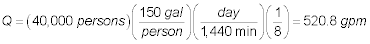
The area of each parallel aeration tray is:
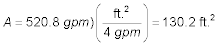
Since L = 2W, then the area of each aerator is A =
W(2W) = 130.2 ft.2
W = 8.068 ft or 8 ft. - 1 in.
L = 2W or 16 ft. - 2
in.
Carbon Dioxide Removal #2
- A groundwater containing 35
mg/L of carbon dioxide is to be degasified using a multiple-tray aerator. The design
population is 150,000 persons, and the maximum day demand is 150 gal/person-day. The
k
value is 0.36, and the hydraulic loading is 3 gpm/ft.2. Determine:
Determine the total number of trays in an aerator required to reduce the carbon
dioxide content of the product water by 90%.
Determine the number of aerators, operated in
parallel, required for the water treatment facility if the size of each
tray is 1,000 ft.2.
Solution: The performance equation is:
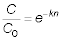
Rewriting the aeration equation to solve for n, the
number of trays gives:
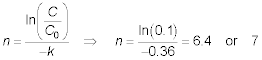
The flowrate to each parallel aerator is:
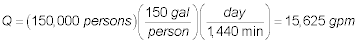
The The total area of all aerations is:
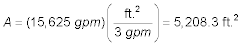
The number of 1,000 ft.2 aerators is:
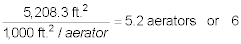
Therefore, six 7-tray (1,000 ft.2)
aerators are required to treat 15,625 gpm.
Disinfection
|
Time,
seconds |
1 |
2 |
4 |
8 |
|
N/N0
|
3,602/10,000 |
1,303/10,000 |
168/10,000 |
3/10,000 |
Solution: The empirical data for the disinfection model should
be plotted with time on the x-axis and ln(N/N0) on the y-axis. The slope
of the line on this plot with give you an estimate of the disinfection constant.
|
Time,
seconds |
1 |
2 |
4 |
8 |
|
N/N0
|
3,602/10,000 |
1,303/10,000 |
168/10,000 |
3/10,000 |
|
-ln(N/N0)
|
1.0211 |
2.0379 |
4.0864 |
8.1117 |
The data are plotted below:

The slope of the line is the disinfection constant.
slope = 8.1117/(8 seconds) = 1.014/sec
The time required for a reduction in cell activity of 1/50,000
is:
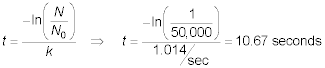
The
contact time required to obtain a reduction of the 1/50,000 of the original number of
virus is 10.67 seconds.
Part 2: Use Excel to develop a table containing the removal of carbon dioxide in a water treatment process using
the aeration model we described in class.
Part 3: Use Excel to
demonstrate the effects of coagulant on effluent turbidity (NTU) as recorded in lab.
-
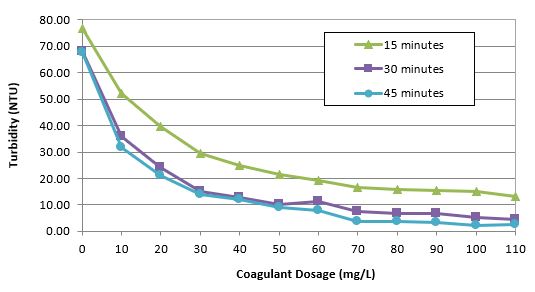
From the jar test
data, plot the supernatant turbidity (NTU) verse the coagulant
dosage. Click
here
for jar test data.
-
Determine the best dosage of coagulant from your
observations and measurements.
-
Using the dosage found in Part b, calculate the quantity
(lb.) of the coagulant needed to treat 20 million gallons per day (MGD).
Jar Test Results
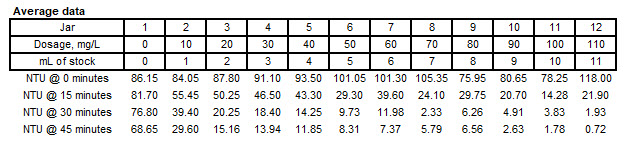
Assuming the you choose 50 mg/L, the amount of coagulant required for
20 MGD is:
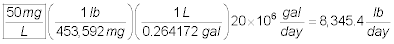
This website was originally
developed by
Charles Camp for
CIVL
1112.
This site is maintained by the
Department of Civil Engineering
at the University of Memphis.
Your comments and questions are welcomed.
|



